In diesem Beitrag vorgestellt
Beyond Paper: Achieving 21 CFR Part 11 Compliance with ERP eSignature Solutions
Teilen Sie
Beyond Paper: Achieving 21 CFR Part 11 Compliance with ERP eSignature Solutions
24 Okt 2025
 Steve Reynolds | Senior Product Manager
Steve Reynolds | Senior Product Manager
In the dietary supplements, nutraceuticals and cosmetics industries, rigorous documentation practices are critical to ensuring product safety, efficacy and regulatory compliance. As companies modernize their operations, electronic signatures (eSignatures) have emerged as an indispensable tool. Under the U.S. Food and Drug Administration’s 21 CFR Part 11 regulations, eSignatures can replace traditional wet‐ink signatures, provided they meet strict technical and procedural requirements.
In this companion blog post to our recent “ERP Perspectives Life Sciences” podcast, you’ll gain practical insights into the foundation of the FDA’s eSignature rules and learn how embedding eSignature functionality into your ERP system can streamline approvals, accelerate audits and strengthen data integrity. Along the way, we’ll cover real world examples, best practices and actionable next steps to help you confidently navigate your digital compliance journey.
What Is FDA 21 CFR Part 11?
21 CFR Part 11 is the section of the Code of Federal Regulations that sets forth the criteria under which electronic records and electronic signatures are considered trustworthy, reliable and equivalent to paper records and handwritten signatures.
Enacted in 1997, its intent is to ensure:
Authentication: Proof that the individual applying the signature is who they say they are.
Integrity: Assurance that the electronic record has not been altered after signing.
Non-repudiation: Guarantee that the signer cannot later deny the validity of their signature.
Part 11 applies to any FDA‐regulated “predicate rule” that requires a signature or recordkeeping, such as Current Good Manufacturing Practice (cGMP) regulations for dietary supplements (21 CFR Part 111) or cosmetics manufacturing.
Who Must Comply and Why Does It Matter?
Like most legislation, there’s nuance to which companies the 21 CFR Part 11 requirements apply to. But if your company does fall under the scope, missing or mishandling eSignatures can create serious compliance risks, slow down approvals, and even jeopardize product safety and legal defensibility. So let’s make sure you’re up to speed.
Who’s Covered?
Manufacturers of dietary supplements under 21 CFR Part 111
Cosmetics companies following FDA guidelines (while cosmetics aren’t subject to as stringent regulations, companies that voluntarily adopt GMPs often apply Part 11 controls)
Contract manufacturers and third-party labs generating records for FDA submission
Any company that electronically creates, modifies, maintains, archives, retrieves or transmits any record required by FDA regulations
Why Is Compliance Critical?
Regulatory Inspections: Auditors routinely assess electronic records and signatures; missing or non-compliant eSignatures can trigger FDA Form 483 observations or warning letters.
Product Safety: Assurance that products are manufactured according to safety standards hinges on traceable sign-off, from master manufacturing records (MMRs) and batch production records (BPRs) to deviation investigations and release approvals.
Legal Defensibility: Proper eSignatures provide a robust audit trail that stands up under scrutiny and reduces liability.
Core 21 CFR Part 11 Requirements
Part 11 may appear technical, but at its heart are a few foundational controls:
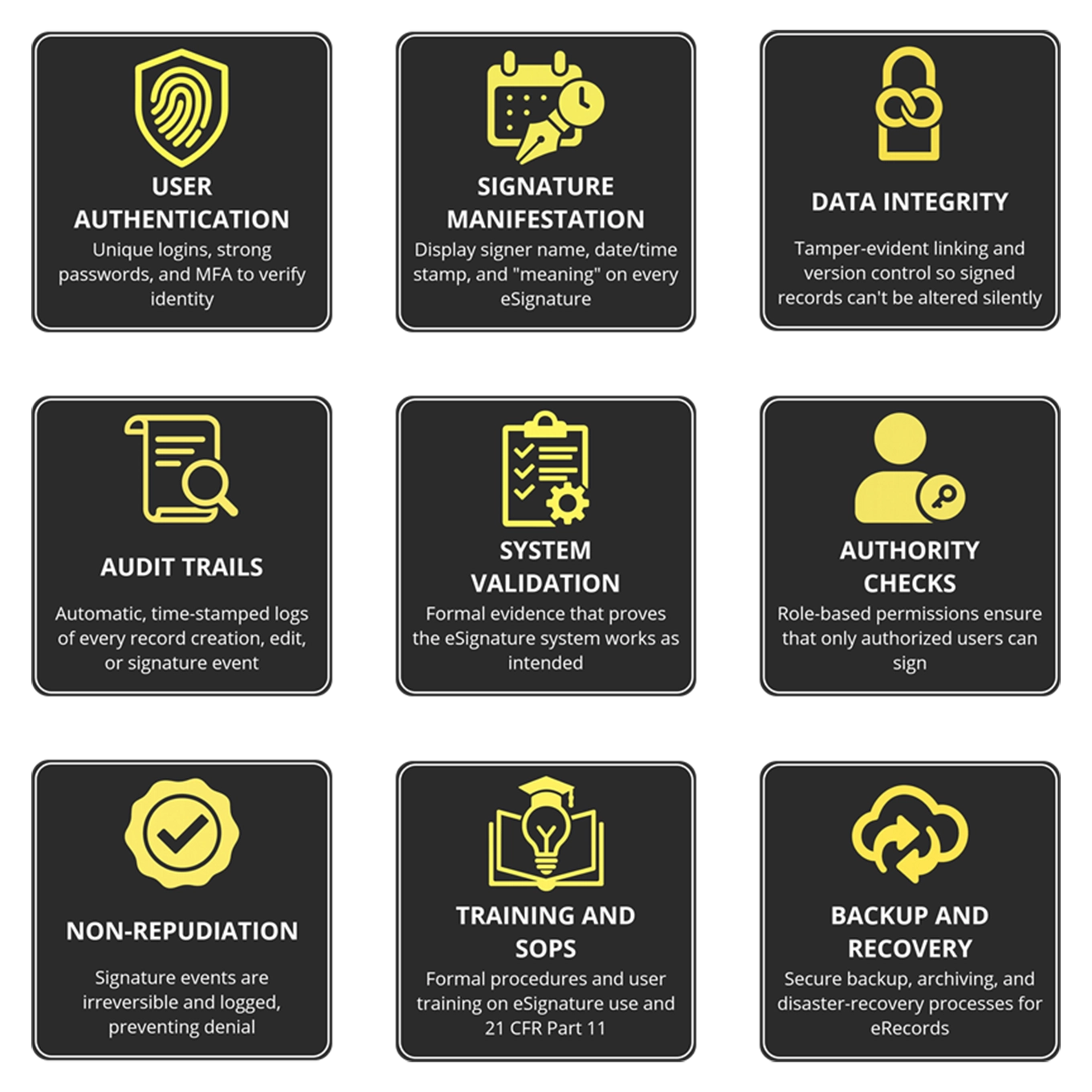
User Authentication: Require unique user IDs and strong multi-factor authentication so every signature is attributable to a real person; integrate with SSO/HR provisioning where possible to prevent shared or orphaned accounts.
Signature Manifestation: Make each eSignature display the signer’s name, role/meaning (e.g., “Approved”), and an exact date/time stamp so records are immediately understandable and defensible in an audit.
Data Integrity: Use tamper-evident linking and version control so any modification creates a new, traceable record, preventing silent edits that can invalidate a signed record.
Audit Trails: Maintain immutable, time-stamped logs of record creation, edits, and signature events, and provide exportable reports for rapid auditor review.
System Validation: Capture IQ/OQ/PQ evidence showing eSignature workflows work as designed under normal and edge-case scenarios; validated systems reduce inspection risk and simplify remediation.
Authority Checks: Enforce role-based permissions and segregation of duties so only qualified, authorized personnel can apply specific signature types, preserving control and accountability.
Non-Repudiation: Bind signatures to records via secure system links and record the signer’s intent so signers cannot credibly repudiate their actions later.
Training and SOPs: Document clear SOPs and train users on eSignature procedures, legal responsibilities, and downtime contingencies; regular refresher training keeps compliance consistent.
Backup and Recovery: Implement secure backup, archiving, and disaster-recovery processes so signed electronic records remain retrievable, intact, and auditable even after system failures.
Implementing these 21 CFR Part 11 requirements is non-negotiable for any regulated electronic record or signature.
The Benefits of eSignatures Over Paper Processes
While Part 11 is driven by compliance, the business advantages of eSignatures make them a compelling long-term investment:
Enhanced Auditability: eSignatures generate comprehensive audit trails that record the who, what and when of every action.
Accelerated Workflows: Automated routing and instant notifications streamline approval cycles, significantly reducing sign-off times and accelerating product release.
Improved Data Integrity: Electronic systems can enforce required field completion and lock records once signed, preventing post-signature alterations and ensuring consistent, error-free documentation.
Role-Based Security: Granular permission settings ensure only designated stakeholders can view, apply or modify specific signatures, strengthening your compliance controls.
Remote and Multi-Site Collaboration: Authorized personnel can securely review and sign documents from any location, supporting multi-site operations and business continuity.
Cost and Space Savings: Eliminating paper usage reduces printing, storage and administrative costs, while electronic archives free up physical space and simplify document retrieval.
The High Cost of Ill-Configured eSignature Systems
While implementing an eSignature policy is an important first step, without properly configured controls, policies and ongoing oversight, organizations risk serious fallout. Below are two real FDA observations that underscore the importance of properly implementing an eSignature system.
Shared Credentials Halt Production
FDA inspectors cited a contract ingredient manufacturer for allowing multiple employees to share a single administrative login for its electronic data system. Because no unique user accounts or secure audit trails existed, the firm could not attribute critical test data entries or edits to any individual. The FDA’s response included:
Production holds until remediation was complete.
Mandated implementation of individual logins with strong password policies and multi-factor authentication.
Deployment of tamper-evident audit trails and validated eSignature workflows under Part 11 controls.
The stoppage and corrective actions cost the company hundreds of thousands in lost output and consultant fees, and sank its customers’ confidence.
Missing Audit Trails in Cosmetics Testing
In February 2024, the FDA found that a contract cosmetics testing lab lacked any reliable audit trail for its electronic assay results. Key findings:
Unrestricted data editing with no record of who made changes or deletions.
No signature linkage, meaning results could be altered without detection.
The FDA deemed the lab’s electronic records unreliable and required the company to:
Enforce role-based permissions to prevent unauthorized edits.
Capture time-stamped audit entries for every record creation, modification and signature event.
The lab faced a months-long suspension of testing services, lost clients to competitors and incurred significant remediation costs.
These examples demonstrate that simply turning on eSignatures isn’t enough. You need a unified system, one that enforces technical controls, ties signatures to real users and provides real-time visibility into compliance. In the next section, we’ll explore how a fully integrated life sciences ERP delivers those capabilities, helping you get it right from day one.
How a Life Sciences ERP Enables 21 CFR Part 11 Compliance
Modern enterprise resource planning (ERP) systems, especially those tailored for life sciences, integrate eSignature functionality directly into core manufacturing and quality modules. Here’s how an ERP supports 21 CFR Part 11 compliance:
Centralized Workflows
Master Records and Batch Records: Create, review and e-sign MMRs and BPRs within the same system where production data resides.
Deviation Management: Record deviations (e.g., “Mixing Vessel Temperature Out of Range”), apply operator and QA eSignatures, and maintain the record in one unified database.
Built-In Part 11 Modules
Pre-Validated Compliance Packages: Many ERP vendors offer out-of-the-box, Part 11–compliant modules that include audit trails, signature workflows and system-level controls.
Vendor-Supported Updates: Regular vendor patches ensure your ERP’s Part 11 features stay current with evolving FDA expectations.
Security and Permissions
Role-Based Access Controls: Ensure only authorized users can prepare, review or approve documents.
Password Policies and Session Management: Enforce strong password rules, multi-factor authentication, and session timeouts.
Audit Trail and Reporting
ERP-Native Audit Trails: Show all user actions and eSignature events, simplifying internal audits and FDA inspections.
Real-Time Dashboard Monitoring: Highlight pending approvals, overdue deviations and open change controls, increasing transparency.
Scalability and Consistency
Multi-Site Deployment: Roll out standardized eSignature workflows across all facilities, ensuring uniform compliance and simplifying validation.
Continuous Improvement: Leverage ERP analytics to identify bottlenecks and optimize eSignature processes.
Implementing Compliant eSignatures in Your ERP
Getting eSignatures up and running “out of the box” is only half the battle. To ensure your ERP-based eSignature solution truly delivers on 21 CFR Part 11 requirements, follow these expanded, step-by-step best practices:
1. Conduct a Detailed Gap Assessment
Compare “as-is” vs. Part 11 Requirements: For each workflow, map signature touchpoints, current document controls (paper routing, physical stamps) and identify missing technical elements (unique logins, audit trails, signature manifestation).
Capture User and System Requirements: Interview end users and auditors to understand pain points (lost forms, incomplete logs) and must-have features (mobile signing, role-based approvals).
Define Success Metrics: Establish clear key performance indicators (KPIs) to measure progress, including approval cycle time targets, zero missing signatures and percent of records with complete audit trails.
2. Validate Early and Often (IQ/OQ/PQ)
Draft a Validation Plan: List all eSignature functions (authentication, signature binding, audit-trail capture) and your test cases for each one.
Perform Installation Qualification (IQ): Verify correct software version, security configurations and network settings for your ERP’s eSignature module.
Execute Operational Qualification (OQ): Simulate typical and edge-case scenarios such as expired passwords, aborted signature sessions, back-dated entries and concurrent user sign-offs.
Run Performance Qualification (PQ): Test day-to-day operations (document creation, signature sequence enforcement, deviation logging) with real users and collect evidence that the system behaves exactly as intended.
3. Document Policies and Standard Operating Procedures
Update your eSignature SOP: Clearly define who may sign what, in what order and under what circumstances.
Establish Contingency Plans: Outline approved paper-backup procedures and how to later reconcile electronic entries, ensuring no gaps exist in your audit trail.
Embed Part 11 Expectations: Include references to specific CFR 11 clauses in your policies (e.g. 11.50 for signature manifestations, 11.10(e) for audit trails) so users understand the regulatory “why” behind each step.
4. Build and Enforce Robust User Management
Unique User IDs and MFA: Disable generic or shared accounts. Integrate ERP logins with your corporate SSO/MFA solution to ensure strong identity proofing.
Role-Based Access Controls: Assign signature roles to users and automate permissions so only those roles see the appropriate eSignature fields.
5. Train, Certify and Empower Super-Users
Hands-on Workshops: Demonstrate the full signature workflow, from document creation through multi-step approvals, so users can practice and ask questions in a safe environment.
Legal-Weight Awareness: Emphasize that clicking “Sign” carries the same legal and regulatory responsibilities as ink on paper.
Super-User Program: Identify 2–3 champions per department who receive advanced training, lead team onboarding and serve as first responders for user issues.
6. Go-Live, Monitor and Support
Phased Rollout: Start with one high-impact area (e.g., batch release), run in parallel with paper if needed, gather feedback, then expand across modules.
Helpdesk and Quick Reference Guides: Provide short “cheat sheets” or reference in-system tool tips so users can quickly recall signature steps and common troubleshooting questions.
Post-Go-Live Audit: Within 30 days of launch, review real audit trails and exception reports to catch misconfigurations (e.g. out-of-sequence signatures, failed multi-factor authentication attempts) and correct them before they grow out of control.
Iterate and Refine: Based on metrics and user feedback, optimize form layouts, adjust workflows or enhance training materials, ensuring your eSignature program matures alongside your operations.
Secure Every Signature: How Aptean Powers Your 21 CFR Part 11 Compliance Journey
As we’ve seen, in your life-sciences business, eSignatures and electronic records aren’t just nice-to-haves: they’re the backbone of regulatory compliance, operational excellence and customer trust. A misconfigured signature workflow or missing audit trail can bring manufacturing to a halt, invite costly FDA observations and undermine your hard-earned reputation.
That’s why you need a partner that you can trust, with tailored software features to solve automate and improve your compliance processes from day one. Aptean’s Life Sciences ERP is designed from the ground up with 21 CFR Part 11 requirements and industry best practices built into every signature step. With our tailored solution, you can:
Automate and enforce unique user authentication, signature manifestation and secure audit trails across your critical processes.
Centralize eSignature workflows for batch records, SOP approvals, deviations and more, ensuring nothing slips through the cracks.
Gain real-time visibility via dashboards and exception reports, letting you spot and resolve anomalies before they become findings.
Scale without compromise. Whether you’re managing a single facility or a global network, you can feel confident knowing every signature adheres to FDA standards.
Backed by deep domain expertise and a dedicated support team, our life sciences ERP empowers your organization to embrace digital signatures. Move beyond paper, reduce risk and keep your operations audit-ready, so you can focus on what matters most: delivering safe, effective products that improve lives.
Get in touch with our team of experts to schedule a personalized demo or listen to our podcast for more life science insights.
Sprechen Sie mit einem Experten für Biowissenschaften
Wir bieten Ihnen die spezialisierten ERP-Lösungen, die Sie für die Herausforderungen Ihrer Branche benötigen.



