Your Starter Guide to MoCRA and What It Means for Your Cosmetic Business
Your Starter Guide to MoCRA and What It Means for Your Cosmetic Business
Your Starter Guide to MoCRA and What It Means for Your Cosmetic Business

If your business is a manufacturer, packer or distributor in the U.S. cosmetics industry, you likely know that considerable changes to compliance requirements are on the horizon with the passing of the Modernization of Cosmetics Regulation Act of 2022 (MoCRA). What you may not know is exactly what the legislation means for your company in terms of necessary adjustments in operations and strategy.
There’s plenty to learn about MoCRA and its implications for your organization—including new records to be created and maintained; testing that must be conducted; and some exemptions that may or may not be applicable given your circumstances—so it’s best to start preparing now. Read on for a high-level overview and general guidance as we enter a new era of cosmetics regulation.
A Brief History of MoCRA
On December 29, 2022, President Joe Biden signed MoCRA into law, marking the beginning of a new paradigm in terms of government oversight and product safety. The bill significantly expands the authority of the Food and Drug Administration (FDA) to enforce standards and protocols for the U.S. cosmetics market.
In large part the result of more than a decade of advocacy, MoCRA includes a number of new requirements for domestic manufacturers, packers and distributors, as well as international businesses that import to the U.S. As the first major reform of cosmetics regulation since 1938’s Federal Food, Drug and Cosmetic Act, many industry experts and politicians alike felt this legislation was a long time coming.
Previously, cosmetic products didn’t need to be approved by the FDA, and recalls of faulty items were not mandatory—but MoCRA reverses course on those policies and entails many additional changes. It also marks the end of the Voluntary Cosmetic Registration Program (VCRP), as cosmetic business registration is now compulsory.
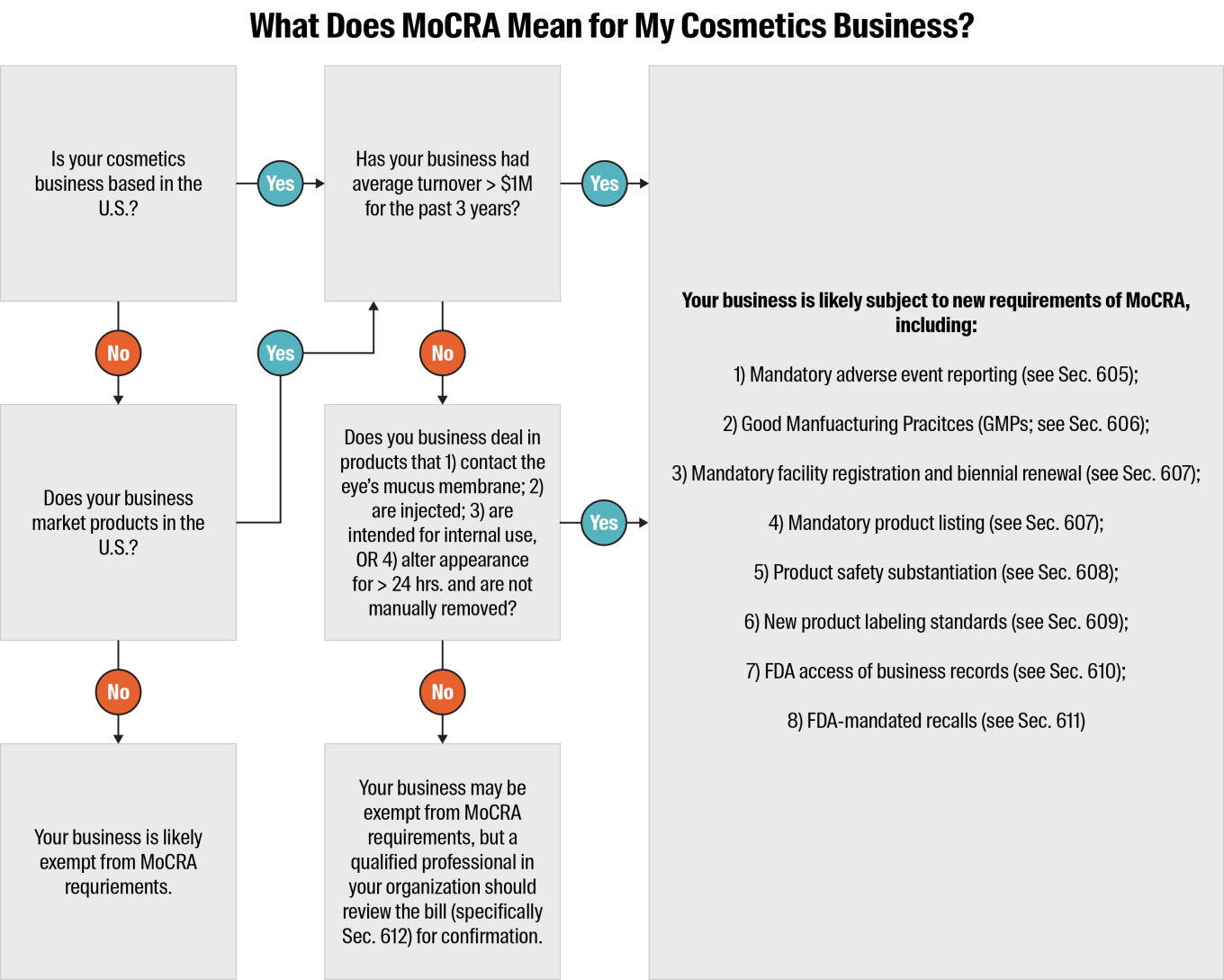
What Businesses Are Subject to the New MoCRA Requirements?
MoCRA and the new requirements it introduces apply to U.S.-based manufacturers, packers, importers and distributors of finished cosmetic products. It also applies to international businesses that market their cosmetic products in the U.S.
There are some exemptions for small businesses with less than $1 million average annual turnover for the previous 3 years. The requirements for facility registration, product listing and Good Manufacturing Practices (GMPs) are waived for these organizations, unless they deal in:
Products that are intended to come into contact with the mucus membrane of the eye
Products that are injected
Products that are intended for internal use only
Products that alter appearance for more than 24 hours and are not normally manually removed
The other MoCRA requirements detailed below would still apply for these businesses.
What Do the New MoCRA Requirements Entail?
With the beginning of MoCRA enforcement rapidly approaching, now is the time for you to read up on the new requirements and prepare for compliance. After all, you don’t want to be caught scrambling when the FDA comes calling for proof of your company’s compliance.
But before we dive in, you’ll also need to understand the meaning of “responsible person” as defined in the law. While the term implies a single individual, it is actually the manufacturer, packer or distributor whose name, address, phone number and electronic contact information appear on product labels.
That said, it is also a good idea for you to determine a qualified staff member who can be reached at that same address and phone number to serve as the main point of contact at your organization for the FDA. You’ll be submitting materials and communicating with the agency more frequently under MoCRA, so it’s best for at least one person to be familiar with the requirements and related records.
With that concept explained, let’s look at the most noteworthy new or updated requirements:
Mandatory adverse event reporting – A responsible person must report serious adverse events (as defined by the bill; includes death, infection, disfigurement and more) to the FDA within 15 days of the incident. They must also provide updated medical information within one year of their report. The FDA has authority to access adverse event records during inspections (see Sec. 605 for more details).
Good Manufacturing Practices (GMPs) – All cosmetics businesses must follow the industry-specific GMPs as established by the FDA (see Sec. 606 for more details).
Mandatory facility registration with the FDA and biennial renewal – All cosmetics businesses must register each of their facilities with the FDA and renew registrations every two years. The FDA has authority to suspend a facility’s registration if the agency determines that cosmetics products manufactured or processed there have a reasonable probability of causing serious adverse health consequences (see Sec. 607 for more details).
Mandatory product listing with the FDA and annual updates – A responsible person must provide to the FDA a detailed account of each of their business’s cosmetic products, including ingredients, and provide annual updates (see Sec. 607 for more details).
Product safety substantiation – A responsible person must maintain records that demonstrate their business’s products are safe for use (see Sec. 608 for more details).
Product labeling standards – All cosmetics products must bear a label that includes the business’s domestic address, phone number and electronic contact information, as well as disclosure of any fragrance allergens included in the product (see Sec. 609 for more details).
Access to business records – If the FDA has a reasonable belief that a cosmetic product is adulterated, or its use could result in serious adverse health consequences, the agency has the authority to access and copy the business’s records associated with that item (see Sec. 610 for more details).
Mandatory recall authority – If the FDA determines that a cosmetic product is adulterated, misbranded or could cause a serious adverse health consequence through use with a reasonable level of probability, it has the authority to order a mandatory recall if the responsible business does not initiate one voluntarily (see Sec. 611 for more details).
Standardized asbestos testing for products containing talc – The FDA has committed to providing standardized asbestos testing methods for products containing talc within one year of the bill’s passage (see Sec. 3505 for more details).
Re-evaluation of the use of PFAs in cosmetic products – The FDA has committed to re-examine the safety risks of using perfluoroalkyl and polyfluoroalkyl (PFAs) in cosmetic products together with the National Center for Toxicological Research and provide additional guidance within three years of the bill’s passage (see Sec. 3506 for more details).
Additional information on enforcement, penalties and pre-emptions, as well as general guidance on product testing on animals, is also included in the bill. Be sure that a qualified individual at your organization studies it closely to determine exactly how all these provisions will impact your company.
When Does MoCRA Go Into Effect?
As is often the case for sweeping regulatory reforms, the FDA is using a phased approach for enforcement so that cosmetics businesses have time to prepare to meet the new MoCRA requirements without immediate threat of penalty. It’s worth re-emphasizing the importance of getting ready now, rather than later—and we have a few general recommendations along those lines.
Starting July 1, 2024, all applicable businesses will need to register their facilities and submit product listings with detailed information. Businesses that began manufacturing cosmetics after December 29, 2022, are exempt, and products that were first marketed after that date are also exempt. Your company can start by assembling the necessary materials and facts, and it’s also a good idea to get underway on safety substantiations.
Starting December 29, 2024, all applicable businesses must disclose known allergens in their products and provide the contact information of their designated responsible person. For now, your key personnel can review FDA hearings on fragrance allergen disclosures and evaluate candidates to act as the responsible person.
Starting December 29, 2025, all applicable businesses must provide proof of adherence to GMPs. Reviewing what those practices entail, as well as the related hearings of the FDA, should help your organization understand what is necessary to satisfy this requirement.
Updates to the law, including those on asbestos testing and the use of PFAs mentioned above, will likely be disseminated in the coming months and years, so make sure your compliance professionals on staff stay tuned. We’ll also be covering additions and changes to these requirements over time as part of our commitment to the cosmetics industry and our customers.
The Importance of Digital Transformation for Regulatory Compliance
In the near future, we’ll be providing additional FAQ content to explore the implications of MoCRA further, and we’ll also share how purpose-built cosmetics product lifecycle management (PLM) solutions—like our own Aptean PLM Lascom Edition—can help cosmetics businesses comply with the new requirements.
For now, we’d simply like to share why digital transformation is important for any business facing compliance concerns. With a cohesive, centralized tech stack, your company can achieve the high degree of data availability and accessibility that the demanding new standards set by MoCRA require.
Your organization can also operate more efficiently, using integration with manufacturing equipment and barcode scanners to automate manual data collection processes. This also helps to reduce the chance of human error, as your employees no longer have to resort to scribbling notes or figures on scratch paper and entering them into the system later.
What’s more, the electronic document management (EDM) features of PLM software and other enterprise systems can facilitate maintaining and locating records that the FDA may request, helping you to quickly provide proof of the compliance of your products and operations.
Aptean is your ideal partner for digital transformation, as our dedicated professionals have deep cosmetics industry knowledge, and we take a collaborative approach to implementation. We also offer flexible cloud deployments of our PLM solution and many more in our full product suite, giving you the scalability, data protection and enhanced cybersecurity you need.
Finally, consider our recent recognition from The Software Report, which ranked us at the 30th overall spot on their Power 500 Software Companies of 2023 list. That’s a testament to the performance, reliability and overall quality of our solutions.
Now, if you’re ready to learn more about Aptean PLM Lascom Edition or any of the other software solutions in the Aptean portfolio, contact us today. You can also request a personalized demo.
¿Todo listo para transformar tu negocio?
Comienza tu transformación con Aptean PLM para empresas de cosméticos y cuidado personal.


 John McCurdy | Senior Content Writer, Marketing
John McCurdy | Senior Content Writer, Marketing